FDA Issues Safety Warning on RF Microneedling Devices: Surgical Risk Signals New Procurement Imperatives
October 15, 2025
The U.S. Food and Drug Administration (FDA) issued a public Safety Communication on October 15, 2025, warning of serious complications linked to the use of radiofrequency (RF) microneedling devices. These instruments—commonly used for aesthetic skin treatments—have been associated with severe injuries, including burns, scarring, skin discoloration, nerve damage, and cases requiring surgical repair.
Although RF microneedling devices are predominantly used in dermatologic and cosmetic practices, the safety alert has sent ripples across the surgical supply chain. Hospitals, outpatient centers, and procurement professionals now face renewed scrutiny over device classification, credentialing, and post-market safety monitoring, particularly as the boundary between aesthetic and clinical treatment continues to blur.
This development signals a broader trend in medical device oversight: non-core surgical devices—including those not typically used in the operating room—can still create serious risk exposures that reverberate through hospital systems, especially when complications escalate to surgical intervention.
In its official statement, the FDA disclosed that it had received a growing volume of Medical Device Reports (MDRs) concerning RF microneedling systems. These devices are designed to penetrate the skin with small needles while delivering controlled RF energy to stimulate collagen and improve skin texture. They are legally cleared under 510(k) pathways for treating facial wrinkles and acne scars—uses categorized strictly under dermatological applications.
However, the agency found that many users—ranging from medical spas to outpatient clinics—have employed these devices in non-approved, off-label capacities, such as body contouring, fat disruption, or deep dermal remodeling. These unregulated applications have resulted in unexpected injuries requiring extensive medical care or reconstructive surgery.
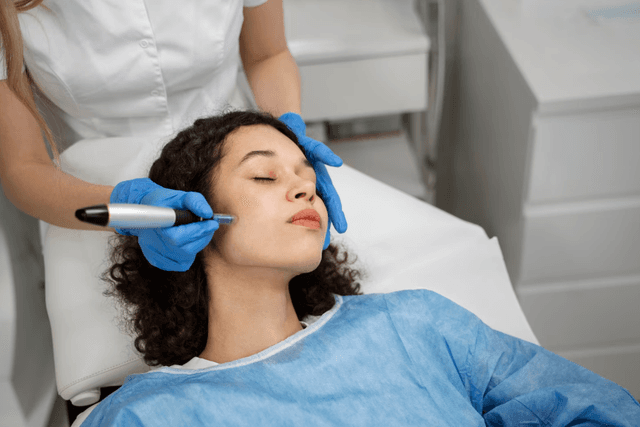
Some patients experienced long-term or irreversible damage, prompting the FDA to advise against certain device applications, and to call on manufacturers to clarify instructions for use (IFUs), restrict marketing claims, and enhance clinician training.
This advisory underscores a growing concern: when devices are used beyond their cleared intent, even minimally invasive tools can create surgical complications. This places increased responsibility on hospitals, ambulatory surgery centers (ASCs), and procurement teams to assess safety documentation, user training, and post-market surveillance metrics when approving new equipment—even outside the OR.
Though these devices are not typically used within traditional surgical workflows, the safety alert has broad implications for the entire surgical supply chain ecosystem, especially in risk management, equipment sourcing, and procedural planning.
1. Increased Post-Market Risk Exposure
Hospitals and surgical centers often find themselves inheriting downstream complications from improperly used outpatient or aesthetic devices. The FDA's advisory reveals that adverse events originating in non-surgical environments may still end up requiring surgical management—placing unexpected burden on OR teams, insurance systems, and hospital liability protocols.
2. Procurement Due Diligence Expands
Procurement and biomedical engineering teams may need to revisit device acquisition policies. Tools that are classified under “non-surgical” categories still warrant rigorous pre-purchase review, particularly when devices are marketed with ambiguous use-cases or off-label flexibility. Vendor claims must be reconciled with FDA labeling to avoid unintentional scope creep.
3. Training and Credentialing Oversight Gaps
The FDA noted that improper training was a contributing factor in many reported injuries. For hospitals contracting out RF microneedling or related services—or where the same provider staff operates multiple device types—oversight on credentialing, continuing education, and manufacturer-certified usage becomes mission critical.
4. Liability and Insurance Ramifications
Hospitals and ASCs must be aware of potential malpractice risks stemming from devices that cross over from cosmetic to surgical categories. Institutions could face litigation or coverage issues if adverse outcomes emerge from tools that were not formally credentialed or disclosed in surgical preparedness workflows.
5. Interdepartmental Coordination Challenges
In cases where surgical repair becomes necessary, communication between dermatology, plastic surgery, general surgery, and procurement teams is essential. Systems should assess whether their adverse event reporting pathways, supply chain feedback loops, and device tracking systems account for cross-specialty device usage.
Hospitals and ASCs: Must evaluate inventory controls, vendor certifications, and IFU compliance for all energy-based skin devices. Adverse event monitoring systems may require updating.